COVID Boosters Set To Revolutionize Treatment Ahead Of Human Testing
Nikki Attkisson | Last Updated : August 30, 2022A new report published by COVID has revealed that several new COVID Boosters will be released to the public later this year, months ahead of their anticipated human testing period. Among these, the COVID Booster set to release first will contain several modifications designed to make it easier to apply and more effective at treating symptoms than earlier models.
This will no doubt come as welcome news to patients who have been eagerly awaiting the arrival of these next-generation medical devices, which are expected to improve treatment significantly in clinical trials currently underway in various locations around the world.
What Are COVID Boosters?
COVID boosters are a new type of treatment that is designed to help the body fight off infection. The treatment is made up of two parts: an antibody and a small molecule.
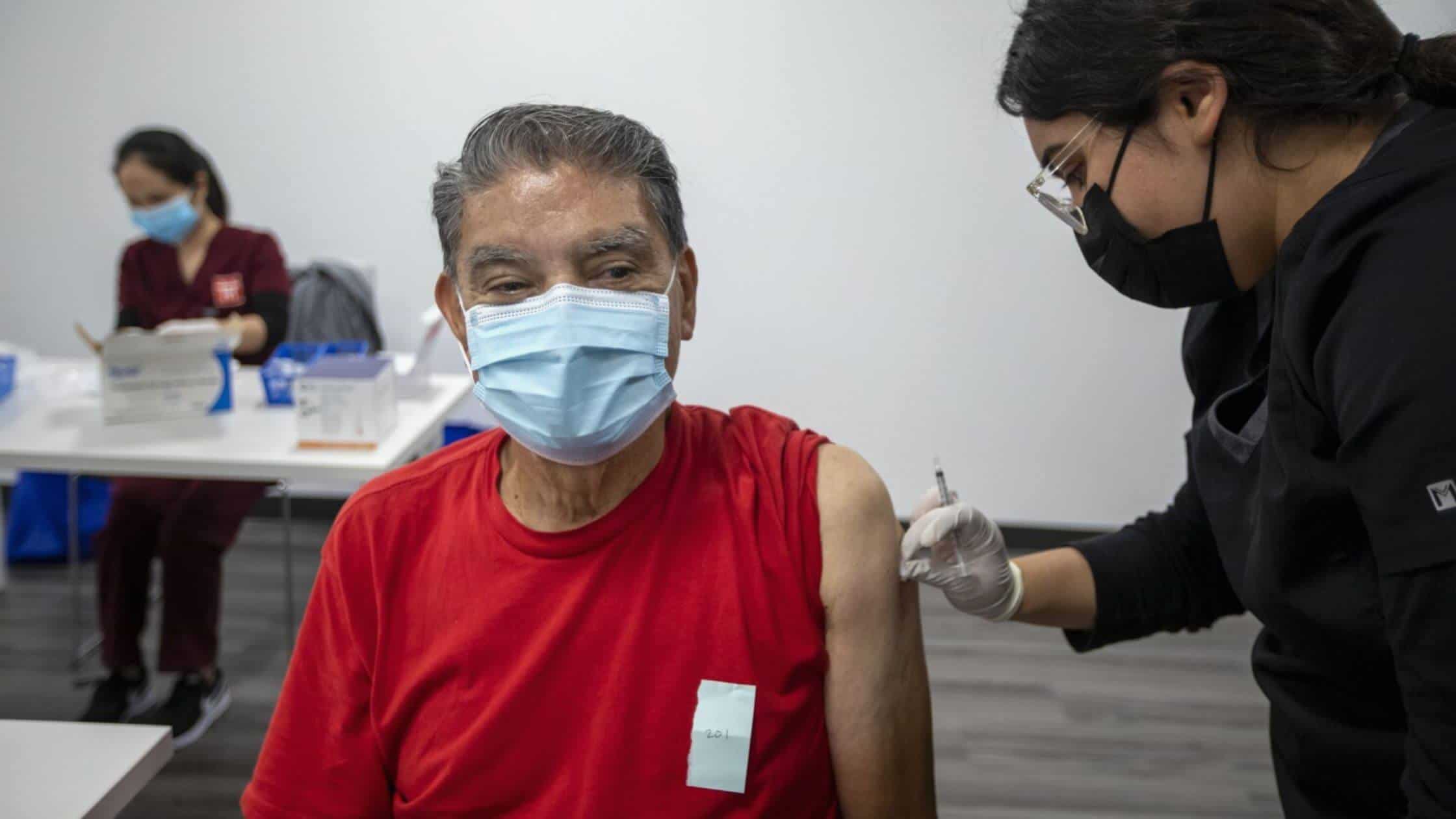
The antibody portion helps the body to recognize and attack the virus, while the small molecule helps to boost the immune system. The treatment is administered through an IV, and it is typically given in three doses over the course of three days.
What’s The Difference Between Early And Late Stage Trials?
Different types of clinical trials are conducted at different stages of drug development. The three main phases of clinical trials are early stage, late stage, and post-marketing.
In early-stage trials, researchers are looking for initial safety and efficacy data on a new drug. During late-stage trials, phase 3 studies assess how effective a drug is compared to standard treatment or placebo.
After drugs have been approved by regulatory agencies like FDA, they may still be subject to post-marketing surveillance which monitors their risks and benefits in large populations over time.
More From PowdersvillePost:
🔵New Zealand: Chiropractic Advice For Fighting COVID This Winter!
🔵Vaccination Boosters For COVID-19 Are Recommended By Your Doctor!
How do boosters work?
The new COVID boosters are said to be even more effective than the current crop of treatments. They work by targeting the virus directly, rather than the symptoms. This means that they can potentially stop the virus from replicating and prevent it from causing serious illness.
The boosters are also said to be less likely to cause side effects, as they target the virus specifically. Human trials are set to begin soon, and if all goes well, these new boosters could be available to the public in the near future.
Benefits of using boosters
As the world awaits human trials for new COVID boosters, the potential benefits of using these boosters are already becoming clear. Boosters have the potential to:
1. Make treatments more effective by targeting specific parts of the virus
2. Help the body build immunity to the virus more quickly
3. Reduce side effects from treatments
4. Make it easier to develop new treatments, as boosters can be used in combination with existing drugs
Other Examples of Medicine That Had Booster Drug Approved First
Although human testing is still required, the Food and Drug Administration (FDA) has approved new booster drugs for COVID-19 that are set to revolutionize treatment. This is similar to how other drugs, such as cancer treatments, have had booster drugs approved first.
The new COVID boosters will help patients better fight the virus and improve their chances of recovery. The approval of these drugs is a major step forward in the fight against COVID.
Conclusion
As the world anxiously awaits the release of new COVID boosters, it is clear that this treatment is set to revolutionize the way we treat the virus. Not only will it be more effective than current treatments, but it will also be much less invasive and have far fewer side effects.
This is a major breakthrough in the fight against COVID and will hopefully help us get the pandemic under control sooner rather than later.
Although we still have a long way to go, it is clear that we’re making some good progress. This is only possible due to hard work and dedication on behalf of scientists around the world.
References:
🔵Center For Disease Control And Prevention(n.d)COVID-19 Vaccine Boosters(Available Online): https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
🔵Australia | Department of Health and Aged Care(n.d)COVID-19 booster vaccine advice(Available Online): https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
With over 15 years as a practicing journalist, Nikki Attkisson found herself at Powdersville Post now after working at several other publications. She is an award-winning journalist with an entrepreneurial spirit and worked as a journalist covering technology, innovation, environmental issues, politics, health etc. Nikki Attkisson has also worked on product development, content strategy, and editorial management for numerous media companies. She began her career at local news stations and worked as a reporter in national newspapers.