The First Fecal Transplant Therapy For Gut Infection Has Received FDA Approval
Nikki Attkisson | Last Updated : December 2, 2022The US Food and Drug Administration had cleared the use of fecal transplant treatment which is known to be an effective therapy in combating antibiotic-resistant gut infections.
Antibiotic resistance is a big challenge that is faced by US health experts and medical researchers as this renders antibiotics useless. The bacterias become immune to these classes of drugs and this pushes healthcare experts to look for other forms of treatment.
Fecal transplant therapy has been around for some time and has been proven effective in fighting against bacteria like Clostridium Difficile popularly known as C. diff.
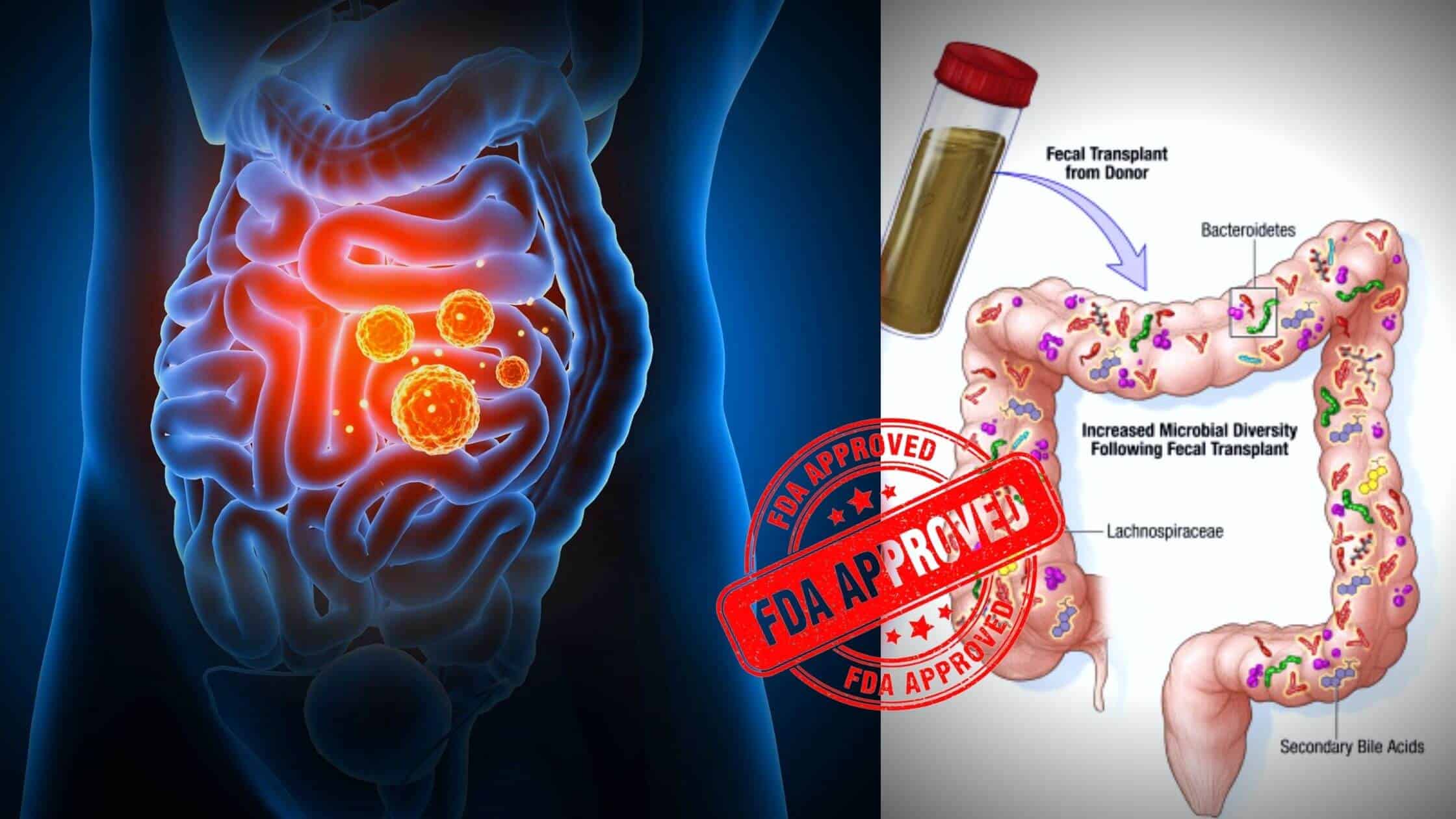
This is the first time that FDA has officially given green light for a fecal microbiota-based treatment. The product called Rebyota is manufactured by Ferring Pharmaceuticals and it has been cleared for use in adults above the age of 18.
It should be used as a successive treatment in individuals after completing the antibiotic treatment for recurrent CDI infection.
It Destroys The Useful Gut Bacterias In Our System
CDI or Clostridium Difficile Infection causes diarrhea and inflammation of the colon. It can be a fatal condition that can cause death too. In the US, the number of CDI deaths can range from 15,000 to 30,000 deaths per year.
One of the main negatives of taking antibiotic drugs is that while they can be efficient in combating an existing infection, it destroys the useful gut bacterias in our system and this can lead to pathogens like C. diff gaining an upper hand.
Rebyota is injected through the rectum as a one-time procedure. It is formulated from the stool of a healthy donor and these stools are passed through numerous tests to identify the presence of any dangerous pathogens.
This has been the risk that has been always associated with the use of fecal transplant therapy and FDA hadn’t given approval in the past for this form of treatment.
In 2019, FDA issued a safety warning against the use of this treatment as one patient had died following the use of a fecal transplant. Two others developed a new infection from the treatment as the stool of the donor had the presence of E.coli, another powerful antibiotic-resistant bacteria that can cause serious infections in humans.
This sort of treatment can also have allergen risks as the fecal matter from a different person’s gut can have allergy-causing substances. More research needs to be done to understand if these allergens can pose danger to the patient.
Even though the fecal transplant treatment has been in use for more than a decade, this is the first time that FDA has approved the therapy. In the clinical trials, it was seen that seventy percent of people who used the Rebyota treatment had their infections resolved in a matter of eight weeks.
More From Powdersville Post:
🔵 The New Method Finds Out Gut Microbes Activate Immune Cells
🔵 A Link Between Gut Health And Autism Spectrum Disorder-Study
Peter Marks, M.D. Ph.D. and director of FDA’s Center for Biologics Evaluation and Research said that the approval of this treatment method can help a lot of people who are struggling with recurrent CDI and also improve their general quality of life.
He considered this approval as an important milestone within the CDI treatment options as antibiotic resistance has become a common cause of concern in the healthcare sector. CDI infection can cause diarrhea, fever, and abdominal pain.
If left untreated, it can affect internal organs and can result in death. Individuals above the age of 65 and people with weak immunity are more at risk. So if you are having any of these symptoms and if they are persisting beyond three to four days, consult a healthcare professional immediately.
These symptoms aren’t just limited to CDI but any sort of infection has to be treated at the earliest.
Reference:
🔵 U.S. Food & Drug Administration (n.d) FDA Approves First Fecal Microbiota Product Available [Online] at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product
With over 15 years as a practicing journalist, Nikki Attkisson found herself at Powdersville Post now after working at several other publications. She is an award-winning journalist with an entrepreneurial spirit and worked as a journalist covering technology, innovation, environmental issues, politics, health etc. Nikki Attkisson has also worked on product development, content strategy, and editorial management for numerous media companies. She began her career at local news stations and worked as a reporter in national newspapers.